What is Ortho Evra?
Ortho Evra is a patch inspired by the 1990s rise of patches used to curb smoking addictions. It came onto the American market in 2002 and is the only patch available currently. The user applies 3 patches over the period of one month and has one week of withdrawal bleeding. It can be applied by the user and only requires a provider to acquire a prescription. It uses both estrogen and progestin to prevent pregnancy. It is currently under fire by many women because of dangers associated with its use but is still on the market in America.
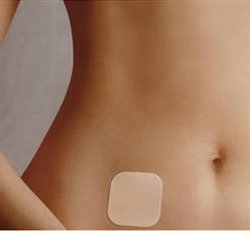
One month’s supply of patches comes with 3 patches, one for each week, aside from the off-week that consists of withdrawal bleeding. The patch has three layers: white-person-flesh colored film (the top layer visible on the skin when applied) that protects the chemicals and adhesive, the sticky part that contains all of the hormones, and the backing removed like a sticker. It can be placed on the arm, shoulder, butt, or abs. It contains 750 micrograms of ethinyl estradiol and 6000 micrograms of norelgestromin (progestin) that are distributed to the body over the period of a week. Research suggests that it may be slightly less effective in heavier women, but the difference is likely negligible.
Ortho Evra is not a very popular option, but it is attractive to women who want something that they don’t need to worry about every day but is still controlled by themselves, not their provider, and does not need to be inserted into the body. Keep in mind that Ortho Evra only comes in beige to match white skin. The patch will be much more noticeable on women of color’s skin tones. I have included this concern of skin color below in my lists.
People who should consider Ortho Evra: women who want a long-term option but are uncomfortable with devices that are provider-controlled, white women who want privacy and ease of use, women who want estrogen, women with heavy periods or bad cramps looking to ease their periods, women who want a hormonal option.
People who should avoid Ortho Evra: women who cannot/will not take estrogen, women who cannot/will not take any hormones, women of color who are uncomfortable with the beige color, women who don’t want monthly bleeding, women who must use lotion all over the body such as women with eczema or dry skin, women who have had or are at high risk for blood clots.
The patch is resilient enough to be worn in water and steam, but is sensitive to other things such as lotions, oil, and cosmetics. Only 5% of patches come loose, but if they do, the user can be susceptible to pregnancy. It must be replaced immediately if it is removed even partially. Women who may often use oil to wrestle or do massages or who model and have make up on various body parts may need to consider another option. Women who must put lotion all over their body for skin conditions should also look further. It is likely that these items would loosen a patch. The good news is that it seems, according to doctors who have studied the patch, forgetting to change your patch is less likely to lead to pregnancy than forgetting a Pill.
Women report side effects with Ortho Evra as with every contraceptive, particularly hormonal ones. Depending on the study you read, 14-20% of women’s skin reacts negatively to the patch. More women report breast tenderness and nausea than Pill users, but they also report less irregular bleeding. This may be explained by the fact that patch users absorb 60% more estrogen overall than Pill users. Women report higher satisfaction with Ortho Evra than oral contraceptives, but also discontinue use at the same rate as Pill users. Early discontinuation is higher in patch users than Pill users.
There has been a lot of call to remove the patch from the market due to blood clot risks and high estrogen levels considered dangerous. Forty women died between 2002 and 2006 because of blood clots while taking the patch. Although it could be coincidental, it is unlikely that there is no connection. There is now evidence that the manufacturer, Johnson & Johnson, neglected to publish two 2001 studies that indicated risk of blood clots that have led to further suspicion and a high drop in prescriptions in recent years.
Next time, I will write about the Pill (both combination and progestin-only pill options). If you have questions about Ortho Evra that I did not answer here, please ask in the comments below or send me a message!


Comments